These snowflakes are actually ice crystals. They form in our atmosphere, high in the clouds, and transform along their journey to Earth thanks to different factors and forces. We look at how snowflakes are formed, and what atmospheric conditions contribute to the beautiful intricacies we’ve come to know them for.
How to Build a Snowflake
The designs of snowflakes are actually products of a crystallization process that is controlled by the atmosphere. Water vapor in the atmosphere latches onto a free-floating speck of pollen or dust and acts as a nucleator. This means that it can begin to add on (ie. nucleate) more water molecules and grow in size. When this happens at cold temperatures, water also freezes and crystallizes. Despite the many unique styles of snowflakes, they all crystallize in the exact same shape—a hexagon. The reason for this has to do with how water behaves at the chemical level. At room temperature, water molecules flow randomly around each other, forming and breaking bonds endlessly. When temperatures cool, however, they begin to lose kinetic energy and form more stable bonds. By 0°C, they reorient themselves into an energetically-efficient position, which happens to be a rigid, hexagonal configuration. This is frozen water, or ice. All snowflakes nucleate and crystallize this way. As more water molecules nucleate to the infant snow crystal, they crystallize long arms and branching tendrils, forming unique, artistic designs. How these designs materialize is simply a matter of water availability and temperature, a relationship best described in the Nakaya Diagram of Snowflakes.
The Nakaya Diagram of Snowflakes
In the 1930s, Japanese physicist Ukichiro Nakaya created the first artificial snowflakes and studied their growth as an analog for natural snow crystal formation. The Snow Crystal Morphology Diagram, or the Nakaya Diagram, is his handy chart that illustrates how snowflakes are formed. The diagram illustrates the kinds of snowflakes that form via atmospheric temperature and humidity during a snow crystal’s fall to the ground. Snowflake size and complexity depend on the humidity of the atmosphere. More water means larger, more intricate snowflakes. Surprisingly, snowflakes cycle between two classes of growth (plates vs. columns) as temperatures decrease. Close to its 100-year anniversary, this detail of the Nakaya diagram still puzzles researchers today. Many continue to theorize and demonstrate how this phenomenon may be possible.
Start the Same, Finish Different
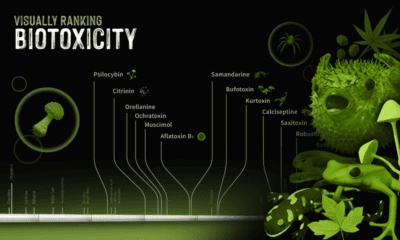
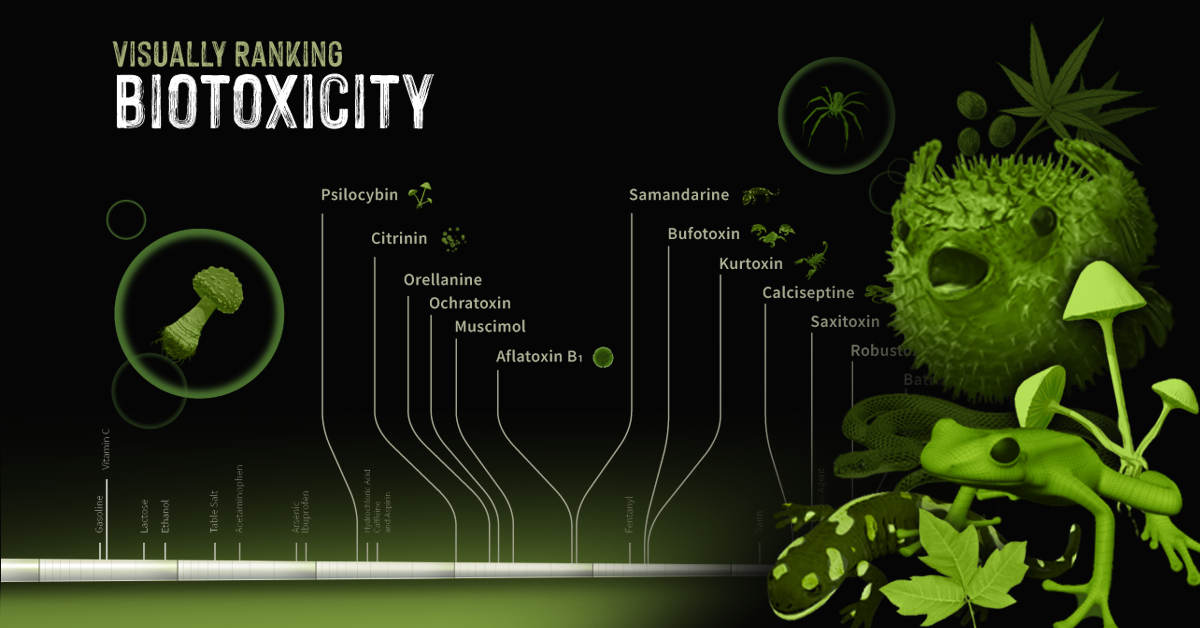
You might be wondering how it is possible that no two snowflakes are identical if they all have a hexagonal inception and can form only columns or plates. The answer lies in the dynamic nature of the atmosphere. The atmosphere is constantly changing. As each second goes by, temperature, humidity, wind direction, and a number of other factors bombard a snow crystal as it falls to the ground. Snow crystals are sensitive to the tiniest of these changes. Water vapor that is crystallizing responds to different exposures which ultimately make new patterns. Since no two snowflakes travel in the exact same path at the exact same time, no two snowflakes will look the same. Same start, different endings. on They can take many forms, from the venom of a snake or spider to the neurotoxins produced by certain types of algae or microbes. In the infographic above, we look at some common biotoxins in the natural world and rank them based on how deadly they are to an average 70 kg (154 lb) human being.
Ranking Biotoxins on a Toxic Scale
A basic concept in toxicology is that “only the dose makes the poison”. Everyday harmless substances like water have the potential to be lethal when consumed in large enough concentrations. Measuring a lethal dosage is very difficult. First, living things are complex: factors like size, diet, biochemistry, and genetics vary across species. This makes it difficult to qualify toxicity in a universal way. Second, individual factors like age or sex can also affect how deadly a substance is. This is why children have different doses for medications than adults. Third, how a poison is taken into the body (orally, intravenously, dermally, etc.) can also impact its deadliness. As a result, there are many ways to measure and rank toxicity, depending on what substance or organism is under investigation. Median lethal dose (LD50) is one common way for measuring toxicity. LD50 is the dose of a substance that kills 50% of a test population of animals. It is commonly reported as mass of substance per unit of body weight (mg/kg or g/kg). In the graphic above, we curate LD50 data of some select biotoxins found in nature and present them on a scale of logarithmic LD50 values. What’s surprising is just how potent some toxins can be.
Bits and Bites about Biotoxins
While one would think that biotoxins are avoided at all costs by humans, the reality is more complicated. Here are some interesting facts about biotoxins present in nature, and our unusual relationships with the organisms that create them:
- Fungi and molds make poisons called mycotoxins Mycotoxins are a global problem. They affect crops from many countries, and can cause significant economic losses for farmers and food producers.
- Phytotoxins can defend plants…and attack cancer Plants use phytotoxins to defend themselves other organisms, like humans. Urushiol, for example, is the main toxic component in the leaves of poison ivy, poison oak, and sumac. But the Pacific yew tree produces taxol that’s valuable in chemotherapy treatments.
- Fire salamander toxin is an ingredient in Slovenian whisky Though not widely available, some whisky makers in Slovenia use samandarine from the fire salamander to create a psychedelic alcohol.
- Ciguatoxins exist in the guts of reef fish Very unique species of bacteria living in the digestive tract of reef fishes make ciguatoxin. They transmit this poison to other organisms when the host fish is eaten.
- Pufferfish are deadly, but also delicious Pufferfish contain tetrodotoxin, a potent neurotoxin in their ovaries, liver, and skin called tetrodotoxin. Despite being a delicacy in many countries around the world, it has a lot of strict regulations because of its ability to kill people. In Japan, for example, only specially licensed chefs can prepare pufferfish for consumption.
- Batrachotoxin is lethal to the touch The skin of some poison dart frogs secretes a deadly substance called batrachotoxin. It is so potent that simply touching the poison can be fatal. Indigenous people of Central and South America used batrachotoxin to poison the tips of hunting weapons for centuries.
- Botox contains the most deadly biotoxin known Commercial botox uses an extremely small amount of biotoxin from a microbe called Clostridium botulinum. It paralyzes the muscles, preventing contraction (i.e. wrinkling). It is the deadliest known biotoxin on Earth. One gram of botulinum toxin can kill up to one million people.
Caveats of Measuring and Reporting Biotoxicity
While we use LD50 data to rank biotoxicity, it isn’t an exact science. There is room for improvement. For starters, no LD50 data exists for humans. That means data from other organisms has to be converted to apply to humans. There is a lot of contention amongst scientific communities about how accurate this is. There has also been an increasing effort to move to new methods of measuring toxicity that are not harmful to animals. Several countries, including the UK, have taken steps to ban the oral LD50, and the Organisation for Economic Co-operation and Development (OECD) abolished the requirement for the oral test in 2001. Now, new ways of evaluating toxicity are under investigation, like cell-based screening methods. Correction: Water was mislabeled on a previous version of the infographic. Full sources here